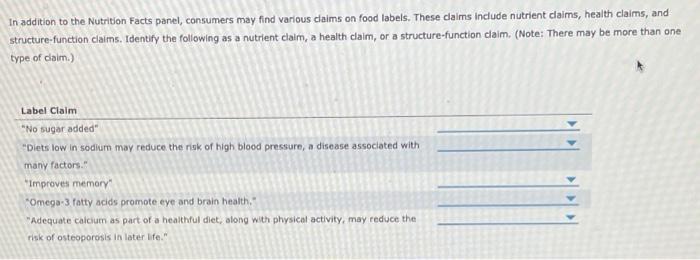
39 structure function claims on dietary supplement labels
PDF Permissible vs. Impermissible Structure/Function Claims for Dietary ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... The FDA permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose ...
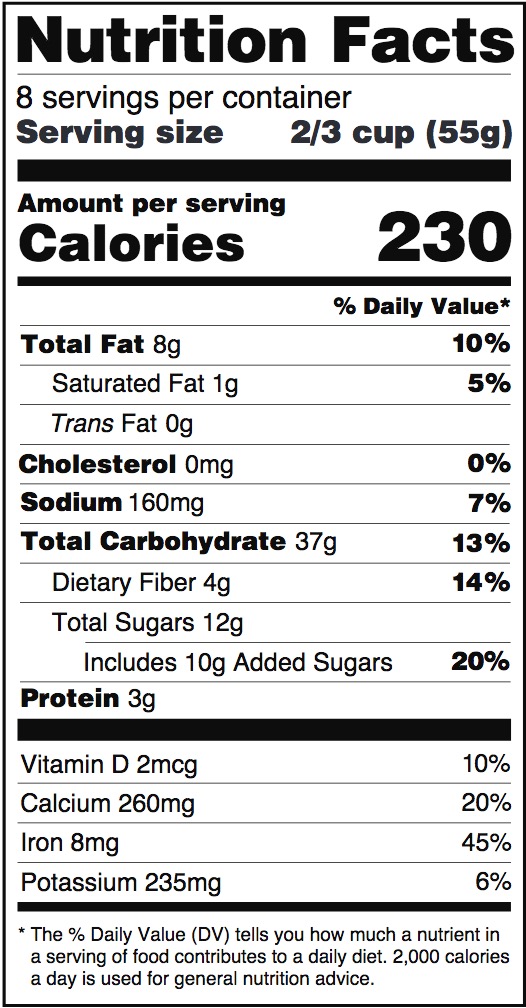
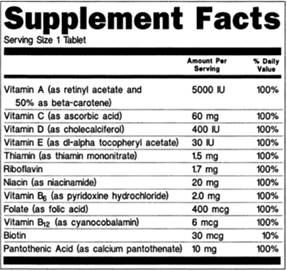
Dietary Supplements Claims, Labels and Regulations | NSF However, they can make other claims on the product label: Health Claims. Disease or health claims show a link between a food or substance and a disease or health-related condition. An example of this type of claim would be, "calcium and a lower risk of osteoporosis" if a supplement contains sufficient amounts of calcium. Structure/Function Claims

Structure function claims on dietary supplement labels
Structure/Function Claims - nutritionlabs.us Dietary Supplements. Structure/function claims have historically appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary ... Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA What health claims can be used on dietary supplement labels? ... What must I do when making structure/function claims in my products' labeling? You must (1) have substantiation that such statement ... Dietary Supplement Health and Education Act of 1994 "(A) the statement claims a benefit related to a classical nutrient deficiency disease and discloses the prevalence of such disease in the United States, describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans, characterizes the documented mechanism by which a nutrient or dietary ingredient ...
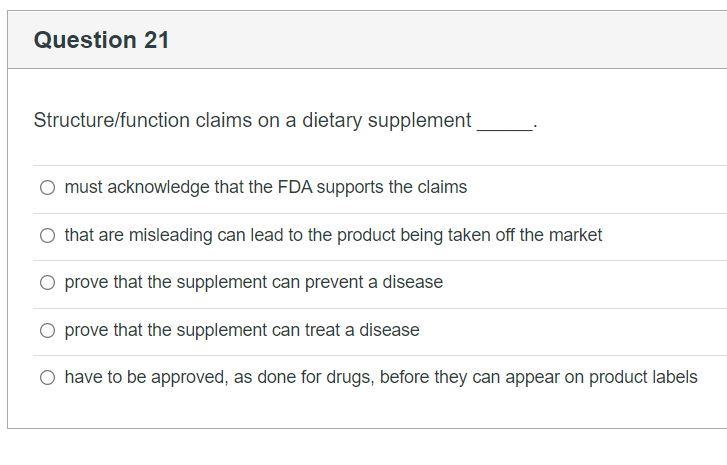
Structure function claims on dietary supplement labels. Should function claims be allowed on dietary supplements? Which claims can or Cannot be made on dietary supplement labels? Basically, dietary supplements cannot make 'disease' claims (for example: 'this supplement shrinks tumors'). Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds ... Understanding Dietary Supplement Claims | Consumer Healthcare ... - CHPA 1. Structure/function claims are the most common, permissible claim used for dietary supplements. DSHEA established special requirements for structure/function claims, including claims that are related to general well-being and nutrient deficiencies. Structure function claims can be easily confused with disease claims, which refer to a specific ... PDF Structure Function Claims: Substantiation Pitfalls A Structure Function Claim A statement describing the effect of a nutrient or dietary ingredient on a structure or function of the human body or characterizing the documented mechanism of such effect. These may appear on the labels of foods, dietary supplements, or drugs. • The definition of what a viable structure function claim varies though Small Entity Compliance Guide on Structure/Function Claims What are structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) ... Dietary supplement labels or labeling may, subject to the ...
Structure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ... Structure-function Claims On Dietary Supplements Labels Exipure, which contains amun and cork bark, is a natural weight loss supplement. Amun cork bark contains an amino acid called amygdalin. Exipure raises brown sugar levels, which is a vitally important dietary factor for burning stubborn belly fat. Drs. James Wilkins and Lam developed Exipure and it is vegetarian-friendly. Label Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Health claims, nutrient content claims, and structure/function claims used on food and dietary supplement labels. Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for using structure/function claims and two related types of dietary ...
Structure/Function Claim Notification for Dietary Supplements Center for Food Safety and Applied Nutrition. Food and Drug Administration. 5001 Campus Drive. College Park, MD 20740-3835. Contact the Office of Dietary Supplement Programs by email at SFCN@fda ... Dietary supplement - Wikipedia Definition. In the United States, the Dietary Supplement Health and Education Act of 1994 provides this description: "The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or ... Dietary Supplement Health and Education Act of 1994 "(A) the statement claims a benefit related to a classical nutrient deficiency disease and discloses the prevalence of such disease in the United States, describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans, characterizes the documented mechanism by which a nutrient or dietary ingredient ... Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA What health claims can be used on dietary supplement labels? ... What must I do when making structure/function claims in my products' labeling? You must (1) have substantiation that such statement ...
Structure/Function Claims - nutritionlabs.us Dietary Supplements. Structure/function claims have historically appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary ...





























Post a Comment for "39 structure function claims on dietary supplement labels"